Explain the Major Differences Between Covalent and Ionic Bonding
Methods of calculation Pauling electronegativity. The maximum oxidation state shown by a p-block element is equal to the total number of valence electrons ie the sum of the s-and p-electrons.

Difference Between Ionic And Covalent Bonds Compare The Difference Between Similar Terms
Pauling first proposed the concept of electronegativity in 1932 to explain why the covalent bond between two different atoms AB is stronger than the average of the AA and the BB bonds.

. Clearly the number of possible oxidation states increases towards. Course Summary Bring the sciences to life for your homeschooler with this high school physical science homeschool course. Our fun video and text lessons act as a comprehensive study guide your.
In the framework of the. We would like to show you a description here but the site wont allow us. And ionic radii ionisation enthalpy etc as well as chemical properties.
In this study we explain this phenomenon by investigating the fundamental electronic differences between water and ammonia the implications of these differences on the PAHwater and PAHammonia interaction and the possible ionization pathways in these complexes using density functional theory DFT computations. Consequently a lot of variation in properties of elements in a group of p-block is observed. According to valence bond theory of which Pauling was a notable proponent this additional stabilization of the heteronuclear bond.
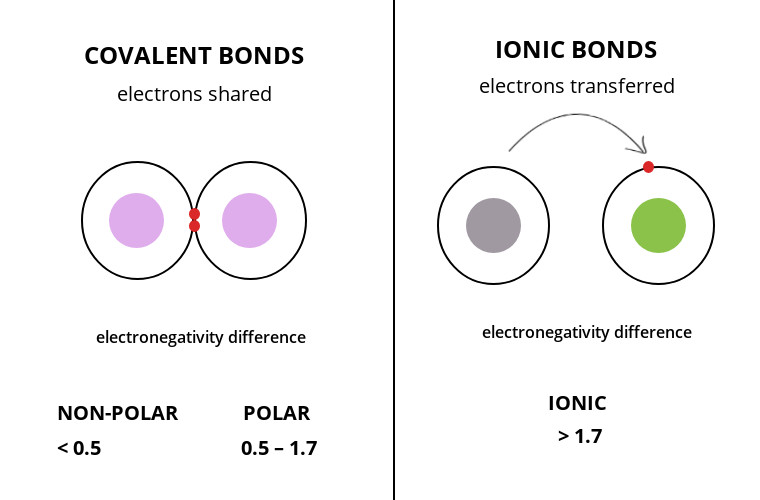
15 Major Difference Between Covalent And Ionic Bonds With Table Core Differences
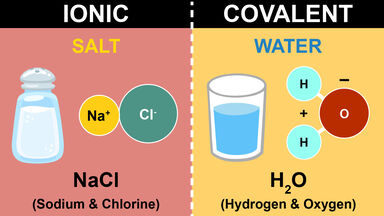
Comments
Post a Comment